
As the electrode reaction for the chemical species in solution occurs on the interface of solution (liquid phase) and electrode (solid phase), in general it proceeds through three elementary steps:
1) substrate mass transfer to the electrode surface;
2) electron transfer reaction on the electrode surface;
3) product diffusion from the electrode.
Electrode reaction rate (current) is considered to be controlled by the slowest process. It is very important to understand the electrode performance controlling process. For example, if the activity of the fuel cell catalyst itself is high enough (the electron transfer reaction is fast), the cell performance can be greatly improved by designing a highly efficient mass transfer electrode (GDE).
Utilizing the rotating disk electrode (RDE) system can control the mass transfer rate for 1) and 3) processes, so that the electrode activity 2) can be quantitatively analyzed in sufficient reproducibility 1-2).
By combining a ring electrode with a disk electrode, the rotating ring disk electrode (RRDE) measurement can help to detect RDE reaction products, their intermediates etc., so that the detailed reaction mechanism can be analyzed3).
In this technical note, while the detailed theoretical formula and analyzing method was omitted, the experiment for RDE & RRDE, such as apparatus using for RDE & RRDE measurement and the basic experimental methods were mainly described. Moreover, the method acquiring the limiting diffusion current data, and Levich and Kouteck-Levich plots preparation method were introduced in brief.
The feature of hydrodynamic voltammetry (RDE, RRDE) is carrying out electrochemical measurement while working electrode is rotating. In order to obtain the mass transfer rate in good reproducibility, it is very important to keep the electrode rotating in a stable speed during the measurement. Hereby, we would like to introduce our new product electrode rotator RRDE-3A Rotating Ring Disk Electrode Apparatus. If RRDE-3A Rotating Ring Disk Electrode Apparatus is equipped with a rotating disk electrode (RDE) (shown in the Figure 1 upper left), the disk working electrode can rotate during electrochemical measurement.
A rotating ring disk electrode (RRDE) is shown in lower left of Figure 1. The disk electrode is in the center position, and a thin insulating layer and a ring electrode locate around the disk electrode in the shape of a concentric circle. When the RRDE electrode is set up to the RRDE-3A Rotating Ring Disk Electrode Apparatus, the rotating ring disk electrode measurement can be carried out easily. If you have the RRDE-3A Rotating Ring Disk Electrode Apparatus, you can do both RDE and RRDE measurements easily.
| The simple specifications for RRDE-3A Rotating Ring Disk Electrode Apparatus are described as follows: 1. Rotation range: 100 ∼ 8000 rpm 2. Rotation accuracy: < 0.1% 3. Power supply: 100 - 240 VAC; 50/60 Hz 4. Operating temperature: 10 - 50°C 5. Inlet gas pressure: 5 psi maximum 6. Size (W x D x H): 190 × 230 × 380 mm 7. Weight: 6 kg |
 Figure 1: RRDE-3A is used for both RDE (Rotating Disk Electrode) and RRDE (Rotating Ring Disk Electrode) for measurements. |

Besides the normal rotation function, the motor portion can be upside down so that the spin coating can be easily done if an acrylic pipe is used together. This function is quite convenience for the electrode modification with catalyst.
Furthermore, gas purge function is equipped in RRDE-3A Rotating Ring Disk Electrode Apparatus, the manual purge time control is available certainly, it works with ALS electrochemical analyzer, and the remote purge time control is also possible.


Electric contact between rotating electrode and potentiostat is performed through the silver carbon brushes contacting to the rotation shaft. Figure 3(a) shows the shaft attached with electrode. The electrode can be removed easily by twisting with fingers. You can see the shaft without electrode in Figure 3(b), and the disk and ring tip parts are insulated by PEEK. The disk tip contacts with the disk electrode, so the disk current goes through the shaft center and flows to the top of the shaft, as shown in Figure 3(c). The ring tip contacts with the ring electrode and the ring current flows to the middle of the shaft. By using two pairs of the carbon brush contacting to the upper and middle parts of the shaft (Figure 4), the ring and disk currents can flow to the potentiostat during electrodes rotating. If the carbon brushes are worn, the noise will occur during measurement. If you find serious noise, please check the carbon brush firstly. If wearing of the carbon brushes is too serious, please replace them.
1) A.J. Bard and L. R. Faulkner. Electrochemical Methods Fundamentals and Applications 2nd Edition, Wiley, New York (2001).
2) K. Oyaizu, M. Yuasa, et al. Electrochemistry, 73, 1060 (2005).
3) K. Oyaizu, M. Yuasa, et al. Electrochemistry., 74, 81 (2006).

The redox reaction with electrode surface electron transfer rate higher enough than the substance diffusion rate is called a reversible system.
[Fe(CN)63−/Fe(CN)64−] is a typical reversible system. In generally, a reversible system for RDE measurement can help to obtain the diffusion coefficient D of substrate, reversible half peak potential E1/2 and electron transfer number n.

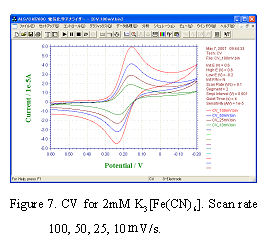
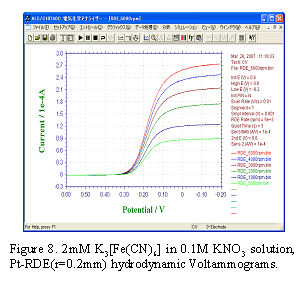

The slow electron transfer process on the electrode surface is called irreversible system (It does not mean the reverse reaction not occurring). An example of the typical reaction is the reduction of dissolved oxygen in acidic solution.
The information obtained from RDE measurement for irreversible system are kinetically-controlled current and the catalyst electron transfer number Napp, which can be calculated from the slope of a Koutecky-Levich plot in the case of electrode catalyst.
Eq.5 is called Koutecky-Levich equation. The first term on the right side of equation is activity determining current which is not dependent on rotation rate. The second term is a reciprocal of Eq.2 and means the mass transfer resistance depending on rotation rate.

4) M. Watanabe, Denki kagaku (presently Electrochemistry),53, 671 (1985).
5) C.Paliteiro, A. Hamnett and J.B. Goodenough, J. Electroanal. Chem., 233, 147 (1987).

RRDE electrode, composed of ring and disk electrodes, is used for RRDE-3A, as shown in Fig.12. The electrode rotation is controlled by RRDE-3A, and both potentials (ED and ER), from ring and disk electrode, are independently controlled by a common reference electrode and counter electrode. The currents are recorded by a dual potentiostat (700C series electrochemical analyzer).
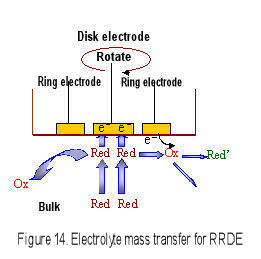
When the RRDE electrode is rotated, the convection occurs near the electrode surface, therefore the diffusion layer thickness is constant and the diffusion limited current is observed. This feature is the same to RDE (Levich equation is used for reversible system). The advantage for RRDE is that the electrolytic product in disk electrode is transported to the ring electrode by centrifugal force and is detected in ring electrode. The electrochemical reaction mechanism in disk electrode is analyzed in more detail.

In order to perform quantitative RRDE measurement, it is necessary to understand the electrolytic species transport situation from disk electrode to ring electrode. The typical parameter is Collection Efficiency N.
If the below reactions occur in disk and ring electrodes, the Red species formed ion the disk electrode are oxidized at the ring electrode, which potential has been set to the Red oxidation potential and can be detected as a ring current.
However, a part of Red species generated on the disk electrode may escape to the bulk during transport from the disk electrode to the ring electrode (Fig.15), and the |iD| > |iR| relation can be found. The collection efficiency is defined as a ratio of the absolute value of ring current to disk current (Eq. 6).
The collection efficiency is a constant determined only by relative configuration and size of both the electrodes and can be calculated using the theoretical equations shown below.
r1 is the radius of a disk electrode, r2 and r3 are the inside and outside radius of a ring electrode, respectively. If r1 = 0.2 cm, r2 = 0.25 cm and r3 = 0.35 cm are used, the calculated collection efficiency is 0.424.
Because an exact electrode surface is uneven, the real collection efficiency is hardly to be coincidence to the theoretical calculation value. In generally, reversible systems such as [Fe(CN)6]4−/[Fe(CN)6]3−, hydroquinone/benzoquinone, ferrocene0/+ , Br−/Br3−and so on are used for RRDE electrode collection efficiency measurement.
Let's see an example for collection efficiency measurement. A Pt ring-Pt disk electrode (r1 = 0.2 cm, r2 = 0.25 cm, r3 = 0.35 cm) was used for RRDE measurement. The RRDE electrode was dipped into 2 mmol/L K3[Fe(CN)6] in 0.1 mol/L KNO3solution and rotated under a rotation rate (e.g. f = 300, 500,……, 5000 rpm). Disk potential ED was scanned from 0.6 V to -0.2 V vs. Ag/AgCl at scan rate 10 mV/s, ring potential ER was fixed to 0.6 V (the reduced product [Fe(CN)6]4- can be oxidized at this potential), and the current-potential voltammograms were recorded during the electrode rotation. Fig.16 shows the RRDE measurement. The ratio of |iR-L| / |iD-L| is almost constant under various ω. The average of collection efficiency N is 0.422, this value is quite near to the calculated N value (0.424).

Pt ring (ID = 5 mm, OD = 7 mm)- GC disk (D = 4 mm) RRDE (polished by alumina and cleaned before use) electrode was set to the RRDE-3A rotator and put into the cell which was equipped with Ag/AgCl reference and Pt counter electrodes. Electrolyte was 1 M NaOH and the saturated oxygen solution was obtained by purging O2 gas for 30 minutes (purge time was controlled by RRDE-3A remote mode). The potential was scanned under static state to understand the proper potential range for the GC disk electrode (potential range changes with the electrode material).

For RRDE measurement, the disk potential scan rate was set to 25 mV/s (depend on experiment, 10, 5 mV/s are also used), the ring potential was set to 0.1 V so that the oxygen reduction product H2O2 formed at disk electrode can be oxidized and detected. The voltammograms were recorded while potential negative scan and electrode rotation. The obtained disk and ring current voltammograms are shown in Fig.18(a) and (b), respectively.

In generally, the oxygen reduction occurred on carbon material in alkaline solution is expressed in above equations. When potential is scanned to negative, the oxygen receives 2 electron (k2) and is changed to HO2-. If the potential is scanned to more negative, furthermore 2 electron reaction (totally 4 electrons) occurs (k3) and OH- is formed, the current increases and the second wave appears. Moreover, the chemical reaction for HO2- disproportionation into O2 and OH- also occurs (k4). Ring potential is set to 0.1 V so that the formed OH- is oxidized completely and detected as ring current.
At -0.5 V disk potential, the disk current of Fig.18(a) increased and the corresponding ring current in Fig.18 (b)also increased. However, when the disk potential was scanned to more negative e.g. -1.0 V, the ring current decreased. This indicated that some of HO2- ion formed on the disk electrode was further reduced to be OH- and caused the ring current decreasing. This means by detection in ring electrode, disk reaction product and reaction mechanism are analyzed. The data in Fig.18 are not so ideal; in reference5) when a Pt ring- pyrolytic graphite (edge plane) disk electrode was used, the second wave 4 electrons reduction proceeded completely, and k1, k2 and k3 were analyzed quantitatively.
In recent years, cathodic catalytic reduction of oxygen relating to fuel cell research has attracted much attention. Platinum or a platinum alloy is often used as electrode catalyst for fuel electrode or oxygen electrode. Research works regarding platinum replacement catalyst are active in order to lower fuel cell development cost6). For example, 4-or 2-electrons oxygen reduction occurred on disk electrode in an alkaline solution. The 2-electrons reduction intermediate HO2- was transported to the ring electrode by rotation and was detected at the ring electrode by oxidation (Fig.19).
Fig. 19 Image for the catalytic reaction occurs on the rotating ring disk electrode.

Hydrodynamic voltammetry RDE and RRDE measurements can obtain the constant current by electrode rotation causing convection near the disk surface and controlling the diffusion layer thickness. Electrochemical reaction mechanism for reversible and irreversible systems can be analyzed by changing the rotation rate. Especially, RRDE can be used for studies of wide range applications7-10) such as fuel cell catalyst reaction, metal, alloy dissolution and plating mechanism analysis, electroorganic synthesis, optical reaction, etc. As the hydrodynamic voltammetry RDE and RRDE can help to acquire much electrochemical information, the much more applications in the future are expected.
5) C.Paliteiro, A. Hamnett and J.B. Goodenough, J. Electroanal. Chem., 233, 147 (1987).
6) H. Meng, P.K. Shen, Electrochemistry Communication, 8, 588, (2006).
7) T. Ousaka, S. Oyama, T. Ohsaka, Electrochemical Methods, Koudansha, Japan (1989).
8) A. Fujishima and K. Honda, Denki Kagaku (Electrochemistry), 42, 213 (1974).
9) M. Watanabe and H. Uchida, Electrochemistry, 68, 816 (2000).
10) K. Tokuda and T. Ohsaka, Denki Kagaku (Electrochemistry), 61, 193 (1993).