
| Variety of Working Electrodes | Features and Applications |
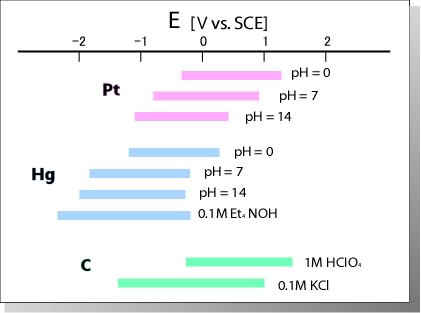
| Platinum electrode (PTE) |
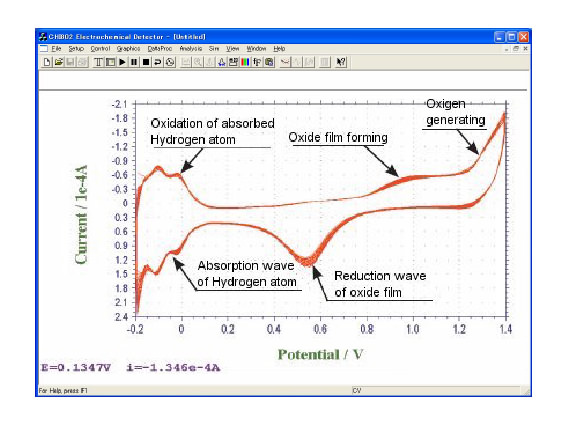
Conventional electrode, which has hydrogen adsorptive wave, used for H2O2 and oxides detection |
| Gold electrode (AUE) |
Conventional electrode, which has no hydrogen adsorption wave, used for thiols detection |
| Glassy carbon electrode (GCE) |
Chemically stable electrode despite its relatively large over-potentials of oxygen and hydrogen evolutions |
| Silver electrode (AGE) |
For cyanide and sulfide detection |
| Carbon paste electrode (CPE) |
Mixed with enzyme etc. to make modified electrodes |
| Nickel electrode (NIE) |
Chemically modified to detect amino acids |
| Palladium electrode (PDE) |
Used to study the process of adsorption and desorption of hydrogen |
| Plastic formed carbon electrode (PFCE) |
Its highly-oriented graphite edge is exposed to the surface with cost effective, similar feature of HOPG |
Our electrodes used for electrochemical measurements such as CV, LSV , DPV and so on are compact and easy enough to be polished by yourself.
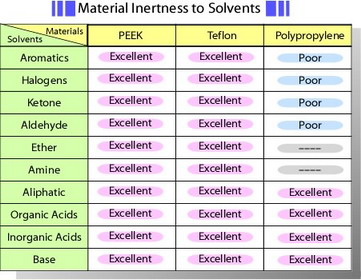
Remove the cap covering the electrode, and check its surface prior to the first use. Carbon paste electrode is available in the form of carbon paste which user packs into a shallow well in the electrode holder supplied by us. Other electrodes are polished to immediate use. These electrodes are encapsulated in PEEK* - solid, well inert against solvent. Table of material inertness to solvents is shown below. However, long-term immersion in tetrahydrofuran or using under higher temperature which exceeds the temperature coefficient between plastic and electrode material might be a cause of crack. O-ring enables a position of an electrode to be adjusted in the voltammetry cell.
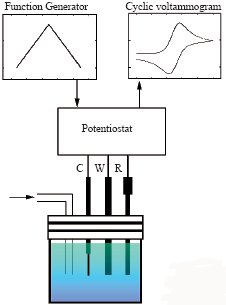
| 1. Little distortion from dissipation in potential or electrification flow 2. Combination of PC enables to process at high sweep rate 3. Analyzable short-lived intermediate in electrode reaction 4. Mearsurable without support electrolyte 5. Excellent measuring accuracy because of little influence of noise arising from electrification flow 6. Smooth diffusion enough to obtain stationary state |
vsAg_Ag_plus.jpg) |
Figure in right : the result of detection of a chemical product in subsequent reaction. By the faster sweep at 104 V/S, the peak (which is shown when the short-lived intermediate is reduced into the former substance) can be observed before the intermediate vanishes. Kosuke Izutsu, Electrochemistry in Nonaqueous Solutions, WILEY-VCH, 2002. |