Spectroelectrochemistry (SEC) is aimed at the investigation of electrochemical reaction mechanism and the interface structure between electrolyte solution and electrode. Remarkable progress in this field and related technology enables SEC to be applied in wide areas.
Nowadays, the relation between absorbance and potential for reversible or quasi-reversible system is theoretically elucidated, on which basis the analysis of electrochemical characteristics becomes possible for the system otherwise difficult with only the result of voltammogram.
Typical example is redox enzyme cytochrome c and methylene blue.
The electrolysis stabilization time for the 0.5 mm optical path length cell is theoretically a half, compared with the 1.0 mm cell. It is the opposite, for the concentration, when the same result for the 1.0 mm cell is possible for a half of the concentration compared with the 0.5 mm cell. You could select the optical path length and the working electrode appropriate for your research purpose.
| Optical path length | Merit | Demerit |
| 0,5 mm | High electrolytic speed | Difficult maintenance |
| 1,0 mm | Easy maintenance | Slow electrolytic speed |
For the comparison of the 0.5 and 1.0 optical path length cells, there is a difference between the theoretical and experimental values. It is in consequence of the experimental conditions.
 |
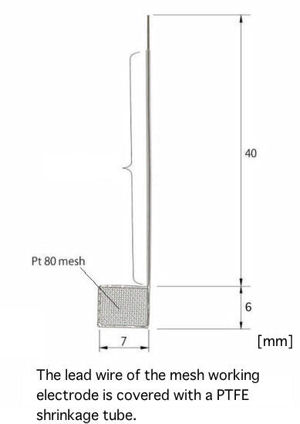 |
The optical path length 1.0 mm is most suitable for basic spectrum electrochemistry measurements. Theoretically, it is possible to get the same result as for 0.5 mm with a half concentration sample.
| Catalog No. | Description |
| 013510 | SEC-C Thin Layer Quartz Glass Spectroelectrochemical cell Kit (Pt) |
| 013511 | SEC-C Thin Layer Quartz Glass Spectroelectrochemical cell Kit (Au) |
| Common Components | |
| 012906 | SEC-C Pt Counter electrode |
| 013512 | SEC-C Thin Layer Quartz Glass cell |
| 011501 | SEC-C Teflon Cap |
| (010537) | Purging tube 10 cm |
| Working Electrodes | |
| 011498 | SEC-C Pt Gauze working electrode |
| 012017 | SEC-C Au Gauze eorking electrode |
| Optional Products | |
| 012167 | RE-1B Reference Electrode (Ag / AgCl) |
| 012171 | RE-7 Non aqueous Reference Electrode (Ag / Ag + ) |
 |
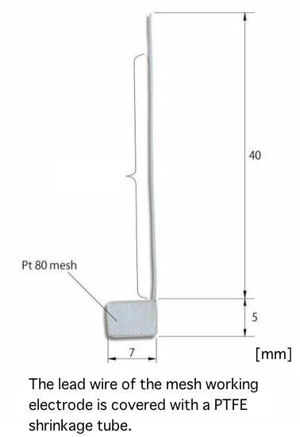 |
The optical path length 0.5 mm has an electrolysis time shorter than 1.0 mm cell. The stability short time for the electrolysis makes possible to have a stable result as for, measurement of the high volatile organic solvent, detection of the unstable electrolysis products, and others.
∗There is a specific working electrode for 0.5 mm optical path length. The working electrode for 1.0 mm optical path length can not be used in 0.5 mm optical path length quartz cell.
| Catalog No. | Description |
| 012813 | SEC-C05 Thin Layer Quartz Glass Spectroelectrochemical cell Kit (Pt) |
| 012814 | SEC-C05 Thin Layer Quartz Glass Spectroelectrochemical cell Kit (Au) |
| Common Components | |
| 012609 | SEC-C05 Pt counter electrode |
| 012815 | SEC-C05 Thin Layer Quartz Glass cell |
| 011501 | SEC-C Teflon Cap |
| (010537) | Purging tube 10 cm |
| Working Electrodes | |
| 012606 | SEC-C05 Pt Gauze working electrode |
| 012607 | SEC-C05 Au Gauze working electrode |
| Produtos opcionais | |
| 012167 | RE-1B Reference electrode (Ag/AgCl) |
| 012171 |
RE-7 Non Aqueous reference electrode (Ag/Ag+) |
UV-visible absorption spectrum and absorbance of the product of the electrode reaction, performed with optically transparent electrode (OTE), were measured. Gold or Platinum mesh electrode was used as an OTE. Cyclic voltammetry and Absorbance of the 2 mM potassium ferricyanide, as the reference of the absorbance, performed in a SEC-C Thin Layer Quartz Glass Spectroelectrochemical cell are shown below (Figure 2-1, 2-2).
  |
  |
|
| Fig.2-1. Cyclic voltammetry for 2 mM potassium ferricyanide. | Fig.2-2. Absorption spectra of the electrolytic balance for 2 mM potassium ferricyanide electrolyzed at different potential. |
Simultaneous measurements of the cyclic voltammetry and absorbance as well a constant potential electrolysis measurement were also performed. The electrolysis, reduction (Figure 3-1) and oxidation (Figure 3-2), of the potassium ferrocyanide solution are shown below.
  |
  |
|
| Fig.3-1. Absorbance changes for the reduction of the potassium ferrocyanide. | Fig.3-2. Absorbance changes for the oxidation of the potassium ferricyanide. |