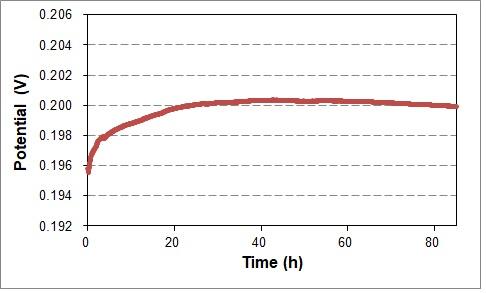
RHE (Reversible hydrogen electrode) was tested for the evaluation, where the stability of the potential in function of the time and temperature was tested and compared with the reference against SHE. Also, the potential difference between RHE and line up reference electrode was measured.
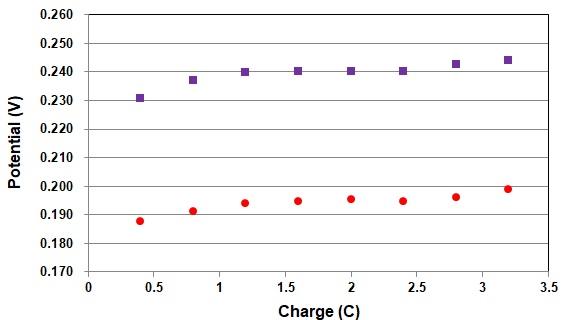
| Experimental conditions | |
| Reference electrode: | RHE Reversible hydrogen electrode |
| Working electrode: | RE-1CP Reference electrode (Ag/AgCl/Saturated KCl) (Cat. No. 013503) |
| EC technique: | open circuit potential - time |
| Solution: | 1.2 mol/L Hydrochloric acid solution |
| Charge passed for hydrogen generation: | 2 C |
| Temperature: | 25°C |
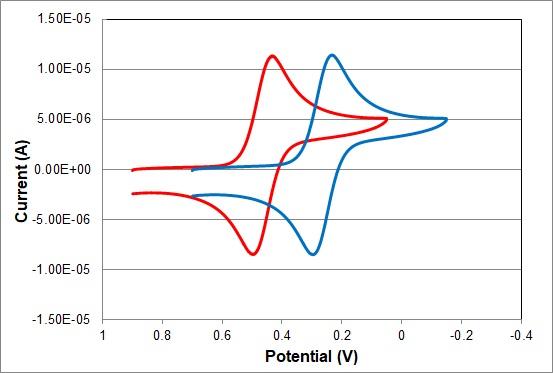
| Experimental conditions | |
| Reference Electrode: | RHE Reversible hydrogen electrode with Double junction chamber and 1.2 mol/L Hydrochloric acid solution |
| RE-1CP Reference electrode (Ag/AgCl/Saturated KCl) (Cat. No. 013503) | |
| Working Electrode: | PTE Platinum electrode OD: 6.0 mm ID: 1.6 mm (Cat. No. 002013) |
| Counter Electrode: | Platinum counter electrode 23 cm (Cat. No. 012961) |
| EC technique: | Cyclic Voltammetry |
| Applied Potential: | 0.9 V ∼ 0.05 V (RHE Reversible hydrogen electrode), 0.7 V ∼ -0.15 V (RE-1CP Reference electrode (Ag/AgCl/Saturated KCl)) |
| Scan Rate: | 0.1 V/s |
| Solution: | 2 mmol/L K3Fe(CN)6 + 1 mol/L KNO3 |
| Temperature: | 25°C |
| Experimental conditions | |
| Reference Electrode: | RHE Reversible hydrogen electrode |
| Working Electrode: | RE-1CP Reference electrode (Ag/AgCl/Saturated KCl) (Cat. No. 013503) |
| RE-2BP Calomel Reference electrode (Cat. No. 013458) | |
| Counter Electrode: | Platinum counter electrode 23 cm (Cat. No. 012961) |
| EC technique: | open circuit potential - time |
| Solution: | 1.2 mol/L Hydrochloric acid solution |
| Temperature: | 25°C |